How Food Ingredients Get The Green Light
If you’ve ever stood in front of the grocery store shelf wondering how so many different ingredients make it into our food supply, you’re not alone. Every ingredient in the U.S. food supply goes through a regulatory framework designed to evaluate its safety before it reaches your home.
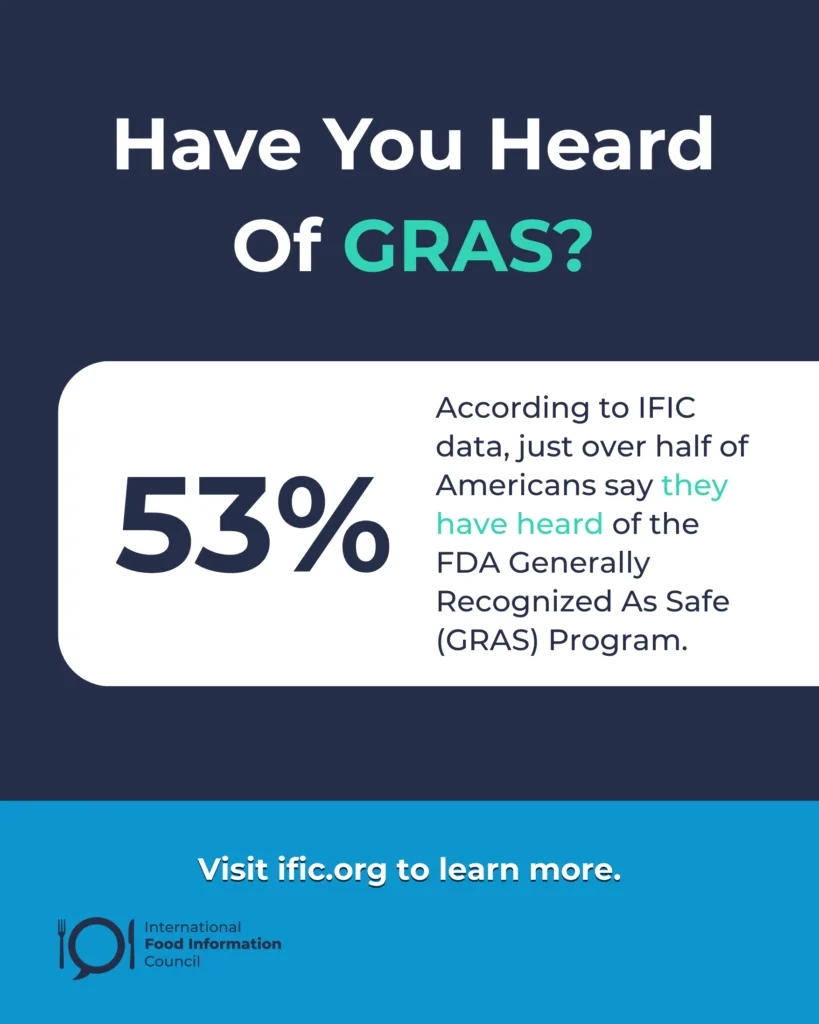
One part of this regulatory system is GRAS, short for Generally Recognized as Safe. If you teach, talk or write about food, nutrition, or food safety, knowing how GRAS works is key to understanding how ingredients are evaluated for safety. It can help you clear up myths with science-based information and make sense of the ongoing debates around transparency in our food system.
Let’s dig into what GRAS is, how it came to be, and how experts can bolster trust in the safety of the U.S. food supply.
A Quick Trip Back In Time: From “Anything Goes” To “Prove It’s Safe”
In the early 1900s, adulteration and contamination of food were not uncommon as testing was limited and resources for enforcing food safety were scarce. The situation began to change with the passage of the Food, Drug, and Cosmetic Act (FD&C Act) and, later, the Food Additives Amendment of 1958. The FD&C Act is a cornerstone of U.S. consumer protection law, designed to protect public health and ensure the quality and integrity of many everyday products. The purpose of the Food Additives Amendment of 1958 was to ensure the safety of ingredients and food additives used in foods in the United States.
Before 1958, ingredients were considered safe until proven otherwise. The Food Additives Amendment flipped that assumption, requiring that most new additives be proven safe before they could be used. It created two main paths for ingredient approval:
- Food Additive Petition (FAP): A formal U.S. Food and Drug Administration (FDA) review of safety data.
- Generally Recognized as Safe (GRAS): A faster pathway for ingredients widely accepted as safe, either by long history of use or strong scientific evidence.
The amendment also assigned the U.S. Food and Drug Administration (FDA) the role of regulating food additives, while Congress retained the authority to change the law itself.
What Counts As A Food Ingredient Or Additive?
Ingredients include everyday items like flour, sugar, and oils; functional components such as vitamins or fiber; and additives used for specific purposes like preserving freshness, enhancing flavor, or improving texture. Under the Federal Food, Drug, and Cosmetic Act, a food additive is any substance expected to become part of food or alter its characteristics, and it is not typically consumed on its own. For example, xanthan gum is a direct additive used to add texture in products like salad dressings and baked goods yet would not be consumed on its own.
The Food Additive Petition Process
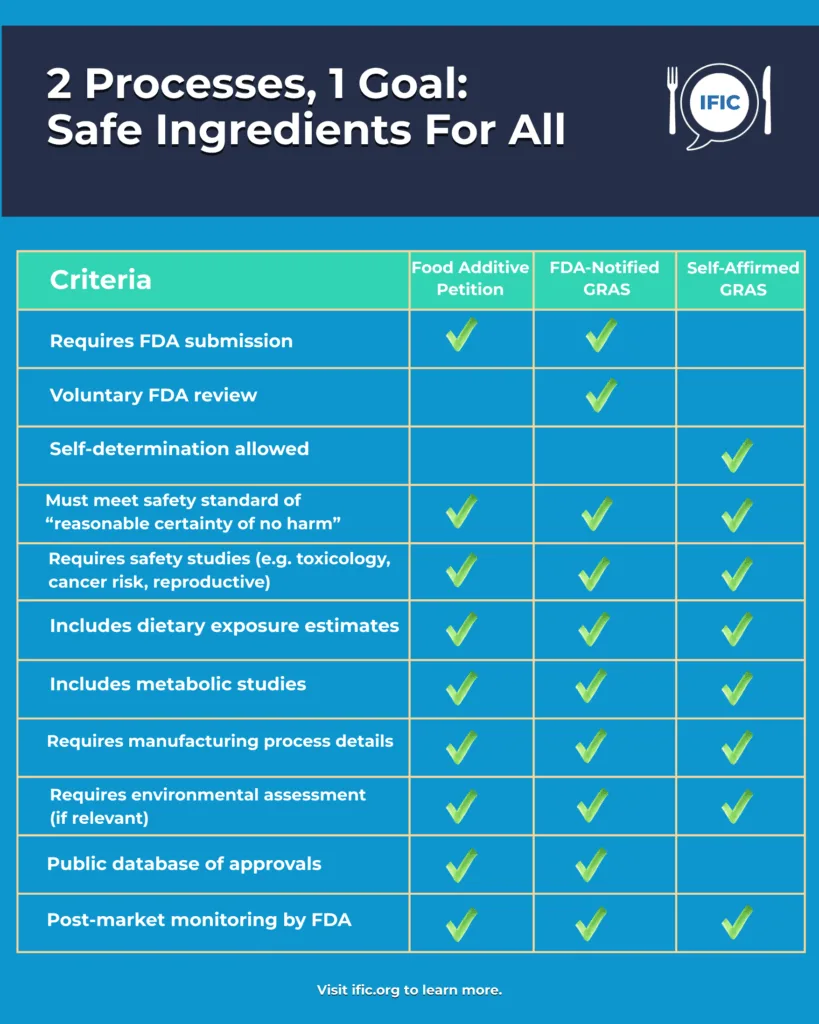
Let’s say a company wants to launch a new food additive or change how an existing additive is used. The company has to send the FDA a Food Additive Petition (FAP). This petition includes lab and human safety studies, ingredient chemical makeup and purity information, how it’s made and where, and data on how much people might consume. The FDA reviews it all to see if it meets the gold standard: reasonable certainty of no harm at expected levels of use. If approved, the ingredient’s official “okay” goes into the Code of Federal Regulations.
While the law requires FDA to decide within 180 days, in practice, reviews often take years because the clock pauses whenever additional data are requested.
The GRAS Process
The 1958 law also carved out GRAS as a special category. There are two ways to qualify for this category:
- Common Use: Safely used in food before January 1, 1958 (e.g. vinegar, vegetable oil, or baking powder).
- Scientific Procedures: Expert consensus based on robust scientific evidence.
There are two ways to gain GRAS status on an ingredient based on scientific procedures. In FDA-Notified GRAS, the company submits evidence to the FDA, which reviews the data and may issue a “no questions” letter if satisfied. With Self-Affirmed GRAS, the company (and often an expert panel) decides the ingredient is GRAS based on the same level of scientific scrutiny, without review or approval by the FDA. There is no requirement to inform the agency that a self-affirmed GRAS determination has been made.
Science Requirements
Whether an ingredient goes through the FAP process or a GRAS review, the safety standard is the same: reasonable certainty of no harm. Key scientific considerations include:
- Dietary exposure: How much people will consume from all sources.
- Self-limiting use: Whether taste, texture, or cost naturally limits how much is used.
- Safety studies: Evidence from animal and human studies on toxicity, DNA damage, reproductive effects, and cancer risk.
- Metabolism: How the body absorbs, processes, and excretes the substance.
- Manufacturing controls: How the ingredient is produced and kept pure.
Importantly, the FDA takes a risk-based approach, considering both the potential hazard and the level of exposure. Some world regions, including Europe, often use a hazard-based approach, restricting or banning substances even if real-world exposure levels are far below any risk.
The Big Debate: Transparency Vs. Trust
The self-affirmed GRAS pathway has come under scrutiny. Critics say it lacks transparency because companies are not required to notify the FDA. They worry about potential bias if safety panels are funded by industry, and about the FDA’s ability to monitor cumulative exposure from multiple products. Supporters argue that manufacturers have strong incentives to use credible science as they risk losing consumer trust, damaging their brand, and facing liability if safety is compromised. Self-GRAS also allows innovation without long delays.
In March 2025, U.S. Department of Health and Human Services Secretary Robert F. Kennedy Jr. told the FDA to consider eliminating self-affirmed GRAS. That would mean all GRAS determinations would go through FDA review.
Congress would likely need to be involved in changing the law, and any reform would need to address how the FDA could handle the increased workload without bottlenecking ingredient approvals as well as adequate funding needs.
The Bottom Line
The FAP and GRAS processes are cornerstones of U.S. food safety, allowing safe, well-studied ingredients to be used in our food supply.
Key Messages For Experts & Educators
For educators and communicators, understanding FAP and GRAS means more than knowing the law and the approval process; it means being able to explain it in a way that builds trust, supports informed choices, and helps bridge the gap between science, policy, and public perception.
The key messages shared below are designed to equip educators and experts with clear, science-based information to address common questions and misconceptions about food ingredients and additives.
The IFIC Spotlight Survey: American’s Perceptions of Food Ingredient Safety found that Americans want more facts (38%) and practical actions (33%) than science (14%) when making food decisions. Whether communicating through social media, educational programs, media interviews, or community outreach, these messages provide a foundation to engage audiences with balanced, accurate, and practical information.
The Role Of Food Ingredients & Additives
- Food ingredients and additives serve important roles in the foods we enjoy every day—helping deliver improved taste, safe storage, appealing appearance and convenience.
- Some food additives help maintain freshness and prevent spoilage, improve texture, enhance color and flavor and allow food to remain safe and affordable.
- Consumers benefit from foods that are more accessible, convenient and consistent without sacrificing safety due to new and innovative food ingredients.
- Novel food ingredients allow for innovation, such as products that meet special dietary needs, support nutrition goals or reduce food waste.
Can I Safely Consume Food Ingredients & Additives?
- Yes, these ingredients are backed by rigorous science and regulatory oversight by the U.S. Food and Drug Administration (FDA).
- Before introducing new additives and similar ingredients into foods, manufacturers invest in strong scientific data to protect human health as well as their brand.
- Communicators can reassure families that the food system is built on multiple layers of safety assessment, monitoring, and regulation designed to protect public health.
How Is Food Ingredient Safety Evaluated?
- Ingredients must be proven safe before use. Whether an ingredient is an everyday staple like flour or a functional component like a preservative, it must meet strict safety standards set by the U.S. Food & Drug Administration (FDA) before being used in food.
- All food substances which are Generally Recognized as Safe (GRAS) must meet the safety standard of “reasonable certainty of no harm,” which is based on comprehensive scientific data including toxicology, dietary exposure, and metabolism studies.
- Food ingredient safety in the U.S. is based on scientific consensus, not guesswork.
- No ingredient can legally be added to food in the U.S. without a safety determination through premarket FDA approval, GRAS status, or a prior exemption.
- U.S. ingredient safety decisions are based on risk, combining both hazard and real-world exposure. This risk-based approach helps ensure food safety while allowing access to a wider variety of ingredients when used within defined consumption levels.
- Consumers want simple, actionable information about ingredients, not complicated science. Educators can build trust by explaining what ingredients do, why they’re used, and how to make informed food choices.
Who Is Responsible For Evaluating Food Ingredient Safety?
- The Office of Food Chemical Safety, Dietary Supplements and Innovation within U.S. Food and Drug Administration (FDA) Unified Human Foods Program oversees the Generally Recognized As Safe (GRAS) and Food Additive Petition (FAP) processes.
- Even after new substances are introduced into foods, FDA monitors ingredient safety and updates approval status as necessary.
- Different countries apply different safety standards—while the U.S. focuses on risk (hazard + exposure), other countries may ban ingredients based solely on hazard using the precautionary principle, even when actual risk is low.
- Food and beverage as well as supplement manufacturers have a fundamental responsibility to ensure the safety of the ingredients used in their food and beverage products as quality and safety are top priorities.
What Is The GRAS Process & How Does It Work?
- GRAS (Generally Recognized as Safe) means that qualified experts, and sometimes the U.S. Food and Drug Administration (FDA), agree a food ingredient is safe to eat based on scientific evidence or a long history of common use.
- GRAS was created to streamline approval of substances with established safety profiles, reducing regulatory burden without compromising consumer safety.
- FDA encourages transparency through voluntary notification and maintains authority to intervene if safety concerns emerge.
- Recent policy discussions are focused on increasing transparency in GRAS determinations, including potential reforms to limit self-affirmed GRAS.
Educators can play a key role in explaining how food ingredients are reviewed for safety and regulated, as well as how the U.S. food system protects public health. For more information, watch the IFIC Expert Webinar: Generally Recognized As Safe Or Generally Misunderstood? Understanding GRAS & Food Additive Safety.
More reading:
Social graphics to share: